November 3, 2025
11 min
Kenneth D
January 15, 2026
11 min

The Underground Healing Molecule Everyone's Injecting
Athletes are injecting it. Biohackers are swearing by it. Your gym buddy probably mentioned it last week. BPC-157—a synthetic peptide derived from a protein in human gastric juice—has become the darling of performance optimization circles, with wild claims about healing everything from torn tendons to leaky gut. Yet here's the catch: despite thousands of Reddit threads and Instagram testimonials, not a single human clinical trial has been published in a peer-reviewed journal.
This creates a fascinating paradox. We've got compelling animal research suggesting legitimate healing properties, an exploding market of people self-administering injections ordered online, and precisely zero FDA approval or long-term human safety data. So what's actually happening here? Let's separate the laboratory promise from the locker-room hype.
BPC-157 stands for "Body Protection Compound-157"—a 15-amino-acid sequence isolated from a protective gastric protein. Researchers first synthesized it in the 1990s at the University of Zagreb, hoping to harness the stomach's natural healing mechanisms. Since then, primarily Croatian and Eastern European laboratories have conducted numerous animal studies exploring its effects.
The research paints an intriguing picture. A 2025 narrative review of BPC-157 for Musculoskeletal Healing evaluated the molecular mechanisms, therapeutic potential, and safety concerns of Body Protective Compound-157 (BPC-157) in the context of musculoskeletal healing. Studies documented that BPC-157 promoted angiogenesis, fibroblast activity, and neuromuscular stabilization, particularly in poorly vascularized tissues such as tendons and myotendinous junctions. They also found it engages ERK1/2 signaling, facilitates endothelial and muscle repair, and exerts anti-inflammatory effects.
Specific animal studies revealed remarkable results. Rats with severed Achilles tendons healed significantly faster with BPC-157 treatment compared to controls. Mice with inflammatory bowel conditions showed reduced intestinal damage. Even brain injury models suggested neuroprotective effects. A 1999 study in the Journal of Physiology and Pharmacology found the compound helped improve orientation, motor function and reduced catalepsy.
But here's where the story gets complicated. Nearly all research comes from a small group of Croatian researchers, primarily from one laboratory. Independent replication remains limited. The studies use varied dosing protocols, administration routes, and animal models, making direct comparisons difficult. Most critically, human trials simply don't exist in the published literature.
The proposed mechanism centers on interaction with growth hormone receptors and modulation of the nitric oxide system. BPC-157 appears to stabilize the extracellular matrix and influence various signaling pathways involved in tissue repair. It doesn't seem to work like traditional growth hormone or steroids—the effects appear more targeted toward healing damaged tissues rather than building new muscle mass.
Methodologically, the research faces several limitations. Small sample sizes, lack of standardized protocols, and publication bias concerns weaken the evidence base. Without human pharmacokinetic data, we don't truly understand absorption, distribution, metabolism, or elimination in people. The optimal dosing remains speculative, extrapolated from rodent studies using questionable conversion methods.
The medical establishment views BPC-157 with deep skepticism bordering on alarm. The Cleveland Clinic and Mayo Clinic both emphasize that this compound lacks FDA approval for any human use. It's classified as a research chemical, not a pharmaceutical drug or dietary supplement.
Dr. Andrew Huberman, a Stanford neuroscientist who discusses performance optimization, has acknowledged the interesting animal research while stressing the complete absence of human safety data. Regulatory bodies remain concerned about unknown long-term effects, potential interactions with medications, and the unregulated nature of available products.
The American College of Sports Medicine maintains that peptides like BPC-157 fall into a gray zone—not officially banned by most sports organizations because they're not well-known enough, yet definitely not recommended or endorsed. Physicians worry about contamination risks with underground products and the precedent of people self-administering experimental compounds based on Reddit advice.
Medical professionals point out that impressive animal results frequently don't translate to humans. The history of drug development is littered with promising rodent studies that failed in human trials. Without proper clinical research, we're essentially conducting uncontrolled experiments on ourselves.
This community sees BPC-157 through a different lens. Dr. Ben Greenfield, a prominent biohacker and wellness consultant, has discussed using BPC-157 personally for injury recovery, viewing it as an extension of the body's natural healing mechanisms rather than a foreign pharmaceutical intervention.
Proponents in this space emphasize that we're working with a synthetic version of a naturally occurring stomach protein. Unlike synthetic steroids or completely novel chemicals, BPC-157 mimics something your body already produces. This argument positions it as amplifying natural processes rather than introducing alien substances.
Integrative practitioners point to the peptide's apparent safety profile in animal studies—no toxicity signals emerged even at high doses. They note that anecdotal human experiences spanning over a decade show consistent injury healing benefits without obvious serious side effects. From this perspective, the regulatory lag represents bureaucratic slowness rather than legitimate safety concerns.
The National Center for Complementary and Integrative Health hasn't specifically addressed BPC-157, but their general stance on peptide therapies acknowledges potential benefits while urging caution with unregulated products. The alternative medicine philosophy generally accepts calculated risks with promising compounds, especially when conventional medicine offers limited solutions for chronic injuries.
Practitioners in this space often recommend working with knowledgeable providers, using pharmaceutical-grade sources, and starting with conservative doses. They view the compound as particularly valuable for injuries that heal slowly through conventional approaches—partial tendon tears, chronic tendinopathy, or persistent gut inflammation.
Scroll through fitness TikTok or biohacking Twitter and you'll find passionate testimonials. Derek from More Plates More Dates, a fitness influencer with over 2 million subscribers, has extensively covered BPC-157, explaining both the potential benefits and regulatory uncertainties. His content represents the more responsible end of influencer coverage—acknowledging unknowns while sharing personal experimentation results.
On Instagram, countless before-and-after injury recovery posts credit BPC-157. Athletes claim torn rotator cuffs healed in weeks instead of months. Bodybuilders report training through injuries that previously sidelined them. Endurance athletes describe bouncing back from overuse injuries with remarkable speed.
Popular posts typically show injection sites, dosing protocols (usually 250-500 mcg once or twice daily), and subjective recovery timelines. The narrative focuses on dramatic healing acceleration, with comments sections full of "where do I get this?" inquiries and source-sharing.
However, critical voices exist too. Some fitness influencers like Jeff Nippard emphasize the lack of human research and caution against blindly following trends. Reddit's r/Peptides forum contains both enthusiastic success stories and cautionary tales about bunk products, injection site reactions, and disappointing results.
The public conversation reveals interesting patterns. People often turn to BPC-157 after conventional medicine offers only "wait and see" advice for soft tissue injuries. The frustration with slow healing timelines drives experimentation. The underground nature creates information silos—knowledge passes through forums and Telegram channels rather than medical literature.
The mainstream medical view and public enthusiasm exist in almost parallel universes. Doctors see unacceptable risk without human trials. Users see unacceptable suffering with standard treatment limitations. This disconnect reflects broader tensions in healthcare—the pace of innovation versus safety validation, patient autonomy versus medical gatekeeping.
All three perspectives acknowledge the impressive animal research. They diverge on what that means for human application right now. The medical establishment says "wait for proper studies." The alternative community says "the body produces this naturally, why wait?" The public says "it's working for me, regardless of studies."
The compounding pharmacy issue unites concerns across all viewpoints. Even enthusiasts worry about product quality. BPC-157 isn't FDA-regulated, so manufacturing standards vary wildly. Testing by independent laboratories has found some products containing little to no actual peptide, others with contamination, and significant potency variations between batches.
One surprising consensus: even advocates don't claim BPC-157 is a magic bullet. Most acknowledge it works best as part of comprehensive recovery protocols including proper rehabilitation, nutrition, and rest. The peptide might accelerate healing, but it doesn't replace foundational recovery principles.
The self-administration concern also creates common ground. Injecting anything carries infection risk, especially without proper training. Stories circulate about abscesses, allergic reactions, and injection mistakes. Even those supportive of BPC-157 use generally recommend medical supervision, though obtaining that proves difficult when most physicians won't touch research chemicals.
1. Properly Designed Human Clinical Trials
The most obvious need involves rigorous Phase I safety studies followed by controlled efficacy trials. We need pharmacokinetic data, optimal dosing information, and head-to-head comparisons with standard treatments. Ideally, multiple independent research groups would replicate the Croatian findings in human subjects with various injury types.
2. Mechanism-of-Action Clarification
We still don't fully understand how BPC-157 exerts its effects. Advanced molecular biology techniques could map its interactions with cellular receptors, identify all influenced signaling pathways, and explain tissue-specific responses. This knowledge would enable more targeted applications and potentially inspire development of related compounds with even better profiles.
3. Long-Term Safety Surveillance
With thousands already using BPC-157 off-label, establishing voluntary registries could track long-term outcomes. Do people develop tolerance? Are there delayed effects? What about interactions with other medications or conditions? Organized data collection beats scattered anecdotes.
4. Standardization and Quality Control
Research into optimal synthesis methods, stability under various storage conditions, and reliable testing protocols would benefit everyone. If BPC-157 eventually gains approval, pharmaceutical-grade manufacturing standards will be essential. Even now, better quality assurance would reduce risks for current users.
5. Comparative Effectiveness Studies
How does BPC-157 stack up against established treatments? Would it complement or replace physical therapy for tendinopathy? Does it outperform prolotherapy for chronic injuries? Could it reduce recovery time after surgery? These practical questions matter more than laboratory mechanisms alone.
BPC-157 represents a fascinating case study in modern medicine's growing pains. We've got remarkable animal research suggesting genuine therapeutic potential for notoriously difficult-to-treat soft tissue injuries and gut conditions. We've got a grassroots movement of people reporting significant benefits from self-directed use. And we've got precisely zero published human clinical trials establishing safety or efficacy.
The compound probably does something. The animal research is too consistent and the anecdotal reports too numerous to dismiss entirely. But "probably does something" falls far short of "safe and effective for humans at these doses." The lack of quality control with available products adds another layer of Russian roulette to the equation.
For those considering BPC-157, the honest assessment requires acknowledging uncertainty. You're participating in an uncontrolled experiment with unknown risks and unproven benefits. The animal research suggests those risks might be low and the benefits potentially substantial, but might and potentially don't constitute medical evidence.
The regulatory system exists for good reasons—protecting people from harmful or ineffective treatments. But it also moves frustratingly slowly, especially for compounds with limited profit potential. BPC-157 can't be patented since it's a known sequence, reducing pharmaceutical company incentive to fund expensive human trials. This economic reality leaves a promising compound in research limbo while injured people make desperate choices.
Perhaps the most valuable insight: healing remains complex and multifactorial. No single compound, no matter how promising, replaces comprehensive injury management. Proper diagnosis, targeted rehabilitation, adequate nutrition, quality sleep, and stress management form the foundation. BPC-157, if it works, would be an adjunct—not a replacement for proven recovery principles.
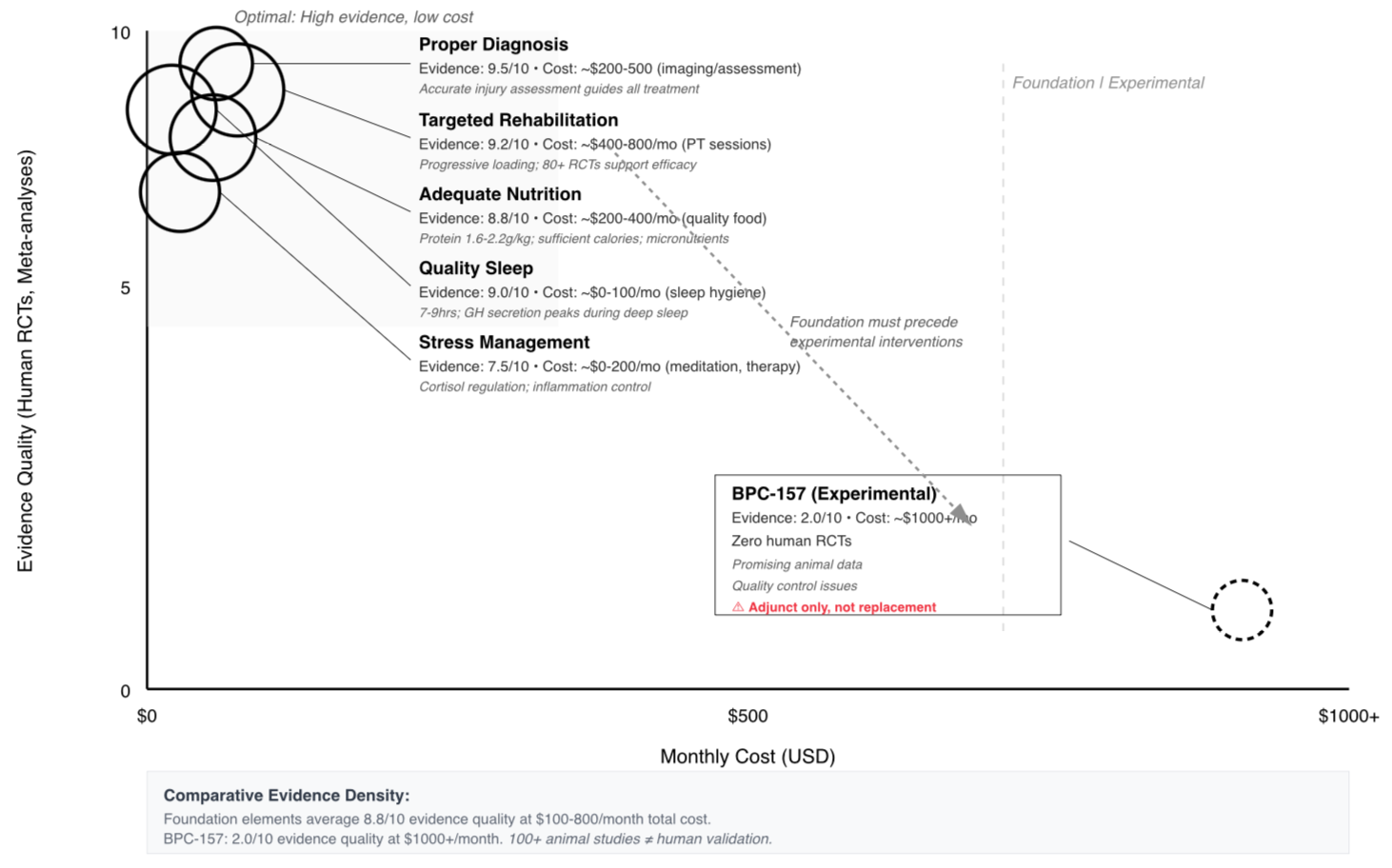
Credibility Rating: 2/10
LyfeiQ Score: 2/10 – The animal research shows genuine promise and deserves continued investigation, but without human trials establishing basic safety parameters and with serious quality control issues in available products, BPC-157 should remain in the laboratory, not your medicine cabinet. The gap between preclinical potential and validated therapeutic application remains vast. Promising does not equal proven.
Disclaimer: This article provides educational information only and should not be construed as medical advice. BPC-157 is not FDA-approved for any human use. Always consult qualified healthcare professionals before considering any experimental compounds or treatments. This content includes personal opinions and interpretations based on available sources and should not replace medical advice. This content includes interpretation of available research and should not replace medical advice. Although the data found in this blog and infographic has been produced and processed from sources believed to be reliable, no warranty expressed or implied can be made regarding the accuracy, completeness, legality or reliability of any such information. This disclaimer applies to any uses of the information whether isolated or aggregate uses thereof.